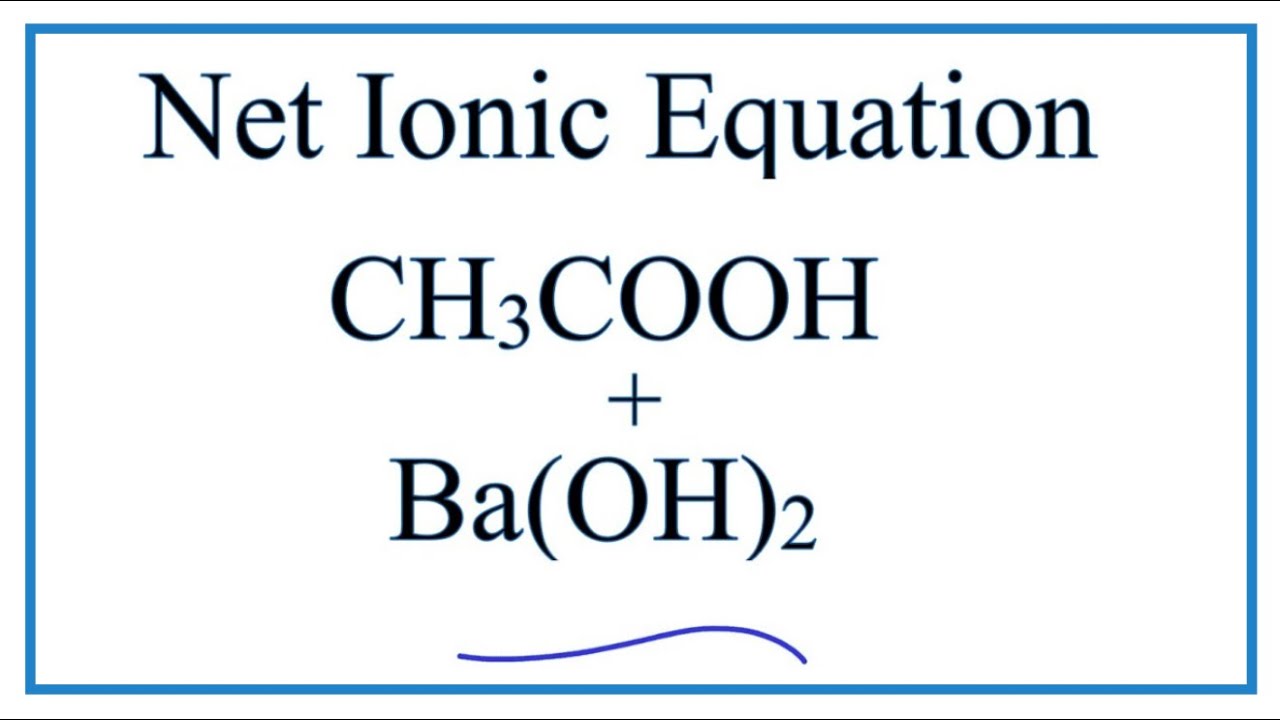SOLVED: Question 10 Not yet answered 100.0 mL of 0.10 M CH3COOH are mixed with 80.0 mL of 0.10 M Ba(OH)2. What determines the pH of the solution after the reaction? Marked

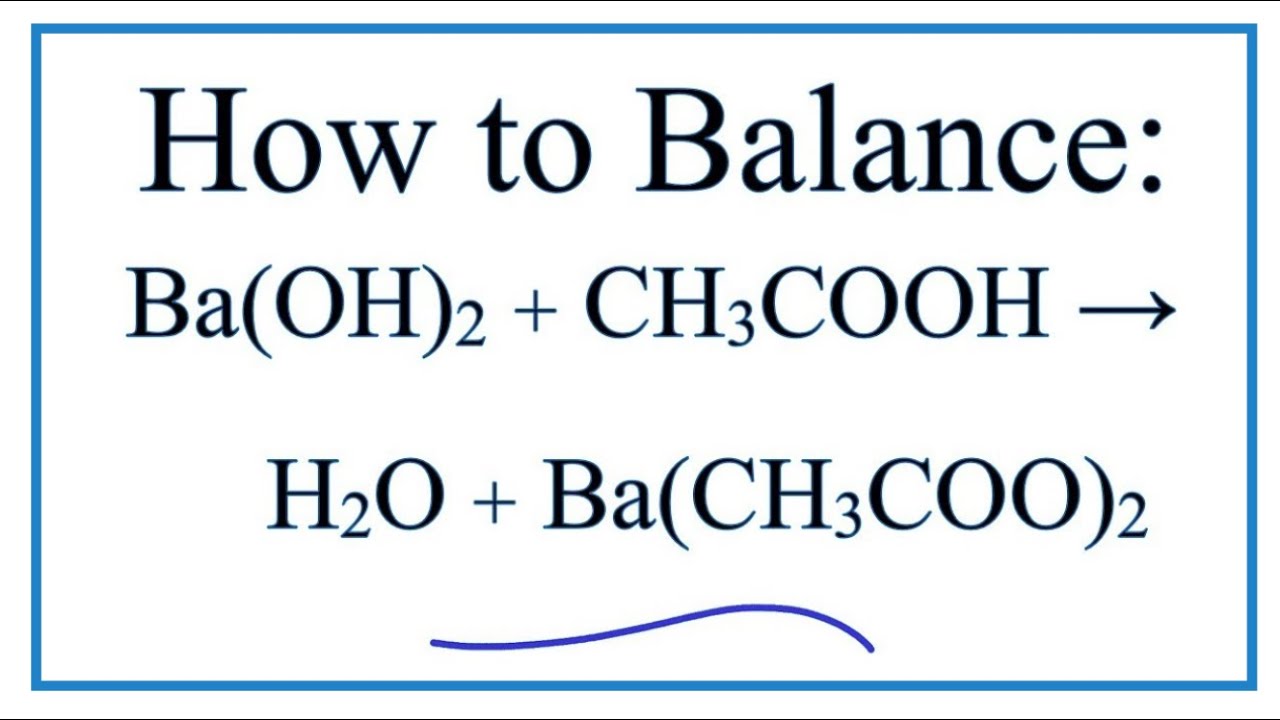
Write the balanced molecular equation for the reaction between aqueous solutions of acetic acid (CH3COOH) and barium hydroxide, Ba(OH)2. | Homework.Study.com

The complete balanced equation for the reaction between barium hydroxide and acetic acid is - brainly.com

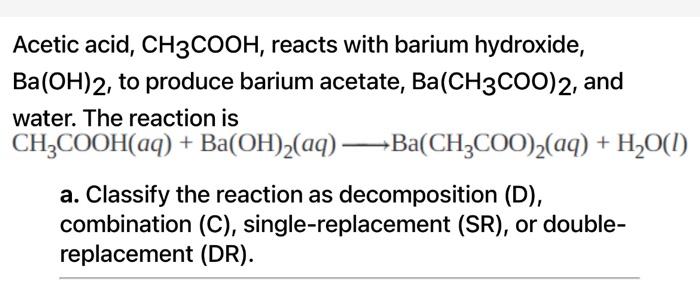
SOLVED: Acetic acid reacts with barium hydroxide. What is the sum of the coefficients for the reactants and products?
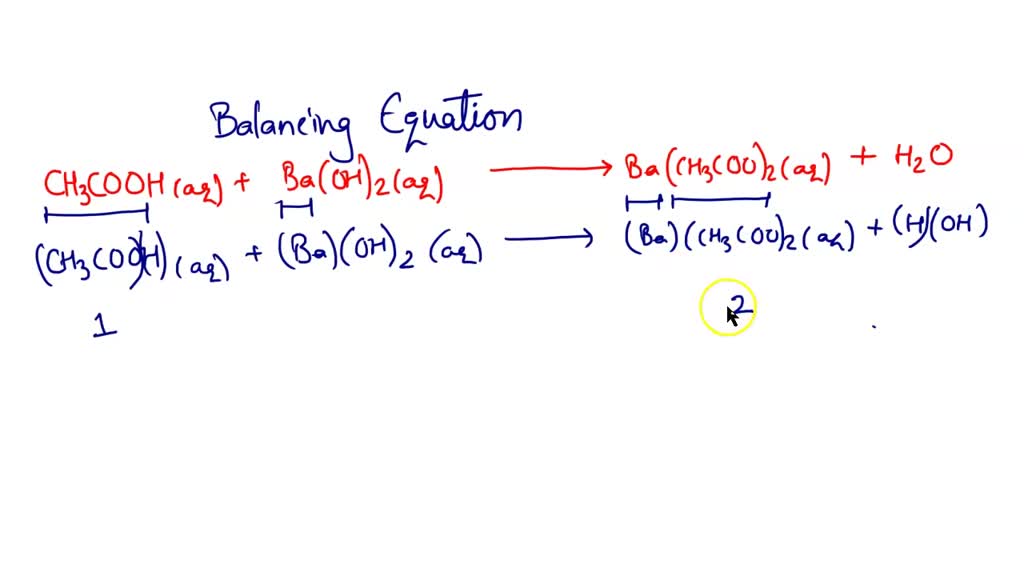
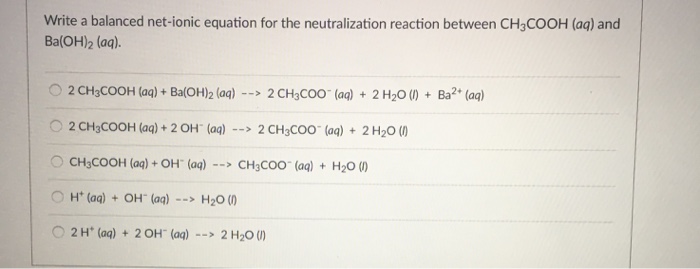
SOLVED: Enter the molecular equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced molecular equation. Identify all of the phases in your answer: CH3COOH(aq) +
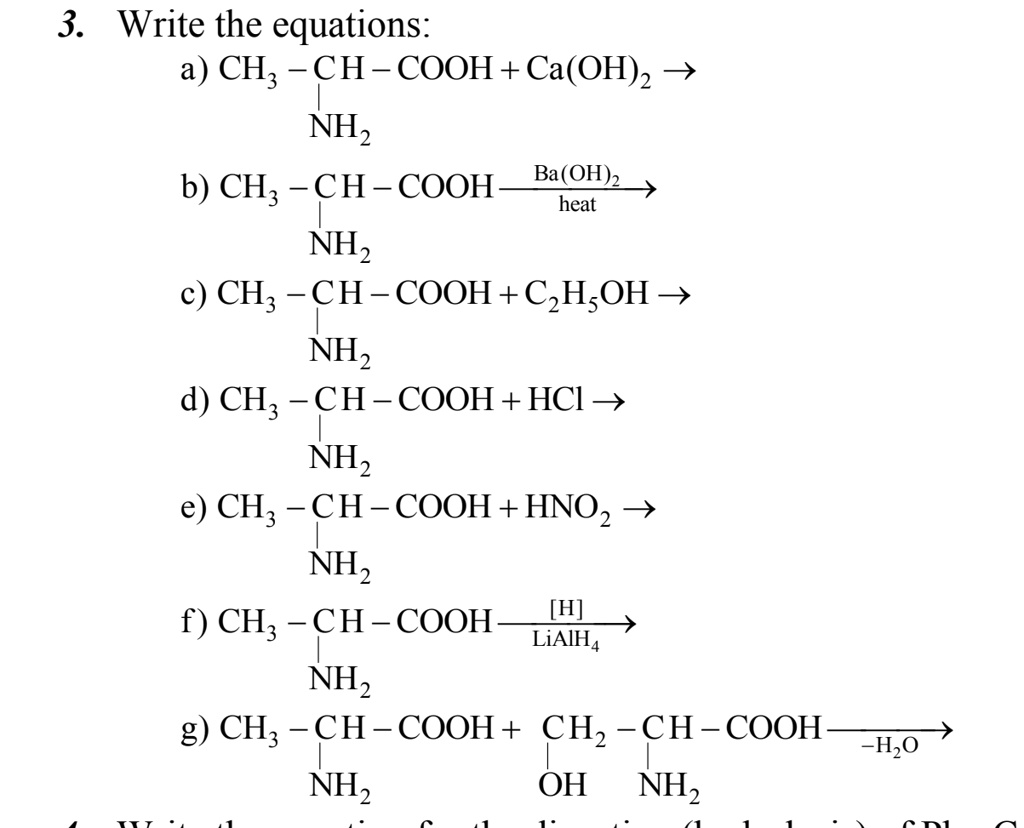
SOLVED: Write the equations: a) CH3COOH + Ca(OH)2 â†' NH2COOH + Ba(OH)2 b) CH3COOH + heat â†' NH2COOH + C2H5OH c) CH3COOH + HCI â†' NH2COOH + H2O d) CH3COOH + HNO2

X(suda) + 2Y(suda) → Tuz + Su Yukarıda verilen nötrleşme tepkimesindeki X ve Y maddelerinin formülü aşağıda verilenlerden hangisi olamaz? Dyod ne ne eigns x Y A) Ca(OH)2 CH3COOH B) H2SO4 NaOH

SOLVED: Enter the molecular equation representing aqueous acetic acid neutralized by aqueous barium hydroxide. Express your answer as a balanced molecular equation. Identify all of the phases in your answer: CH3COOH(aq) +