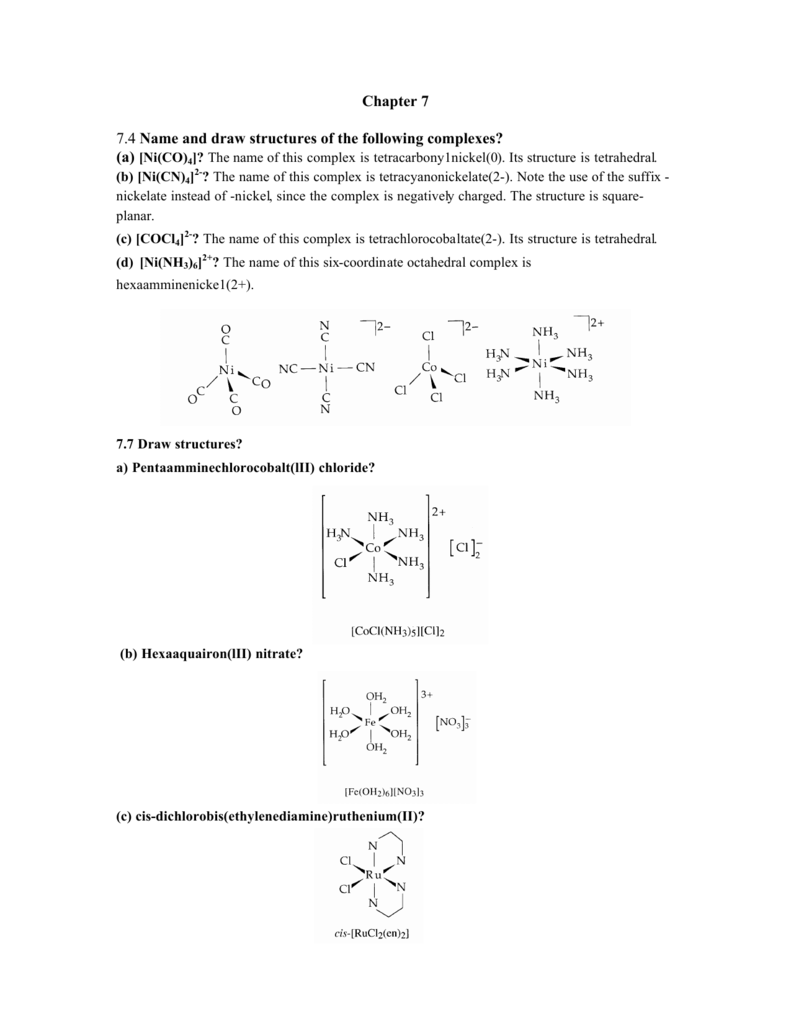
69 The no of geometrical isomers for octahedral [CoCl4(NH3)2 ] , square planar [AuBr2Cl2] , and [PtCl2(en) ] are
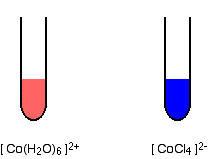
The formation of CoCl4 2- from Co 2+ and Cl- is endothermic. Are the color changes that accompany heating and cooling of equilibrium mixture in accord with Le Chatelier's principle? | Socratic
![Determine the structure and magnetic behaviour of [CoCl4 ]²- using valence bond theory. according to board - Brainly.in Determine the structure and magnetic behaviour of [CoCl4 ]²- using valence bond theory. according to board - Brainly.in](https://hi-static.z-dn.net/files/dbc/4e4b8ae040fa0c9d9e6932ef4496ad25.jpg)
Determine the structure and magnetic behaviour of [CoCl4 ]²- using valence bond theory. according to board - Brainly.in
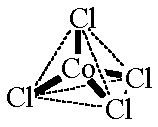
The complex salt $CoCl_{ 4 }^{ 2- }$ has a tetrahedral structure. How many d-electrons are on the cobalt?
Predict the number of unpaired electrons in [COCl4]^2- ion on the basis of VBT. [COCl4]^2- - Sarthaks eConnect | Largest Online Education Community
![SOLVED: What is the correct formula for sodium tetrachlorocobaltate(I)? a. NaZ[CoCl4] b. Na2[CoCl4] c. Na4[CoCl4] d. Na[CoCl4] e. Na[CoCl4] SOLVED: What is the correct formula for sodium tetrachlorocobaltate(I)? a. NaZ[CoCl4] b. Na2[CoCl4] c. Na4[CoCl4] d. Na[CoCl4] e. Na[CoCl4]](https://cdn.numerade.com/ask_previews/9d18200b-36c3-4d2c-904b-4866aaaff2ba_large.jpg)
SOLVED: What is the correct formula for sodium tetrachlorocobaltate(I)? a. NaZ[CoCl4] b. Na2[CoCl4] c. Na4[CoCl4] d. Na[CoCl4] e. Na[CoCl4]
![Answer in brief. [CoCl4]2- is tetrahedral complex. Draw its box orbital diagram. State which orbitals participate in hybridization. - Chemistry | Shaalaa.com Answer in brief. [CoCl4]2- is tetrahedral complex. Draw its box orbital diagram. State which orbitals participate in hybridization. - Chemistry | Shaalaa.com](https://www.shaalaa.com/images/_4:65f664b9fefa456ba348e04d03e2557a.png)
Answer in brief. [CoCl4]2- is tetrahedral complex. Draw its box orbital diagram. State which orbitals participate in hybridization. - Chemistry | Shaalaa.com
![SOLVED: [CoCl4]^2- is a Co^2+ tetrahedral comblex that is colored. 4.2. Explain why this complex is so brightly colored. Complete the sketch of the molecular orbital diagram 4 p≡ provided by adding SOLVED: [CoCl4]^2- is a Co^2+ tetrahedral comblex that is colored. 4.2. Explain why this complex is so brightly colored. Complete the sketch of the molecular orbital diagram 4 p≡ provided by adding](https://cdn.numerade.com/ask_images/a8ab43fa57554054b4968f6b29691ccb.jpg)
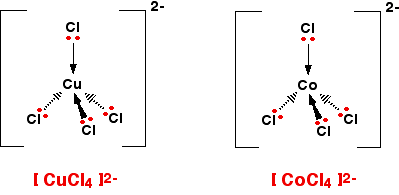
![CoCl4]2- - Cobalt tetrachloride CoCl4]2- - Cobalt tetrachloride](https://www.chemtube3d.com/images/gallery/inorganicsjpgs/cocl42-.jpg)

![Predict the hybridisation and geometry of `[CoCl_(4)]^(2-) and [Co(CN)_(4)]^(2-)` Predict the hybridisation and geometry of `[CoCl_(4)]^(2-) and [Co(CN)_(4)]^(2-)`](https://i.ytimg.com/vi/m7yduxa5Ysw/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLDioezo6yafUtFRdVQZgUlgZXiOWg)

![Solved Question 13 What is the IUPAC name for K2[CoCl4]? A. | Chegg.com Solved Question 13 What is the IUPAC name for K2[CoCl4]? A. | Chegg.com](https://media.cheggcdn.com/study/804/804d85ef-b8b9-4d52-9504-6c7c40d3bef1/image.png)
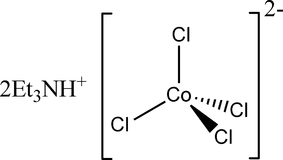
![Write the IUPAC name and hybridisation of K(2)[CoCl(4)(en)] Write the IUPAC name and hybridisation of K(2)[CoCl(4)(en)]](https://static.doubtnut.com/ss/web/1312549.webp)
![Spectroscopic ground state term symbols of cobalt ions in [Co(H2O)6]2+ and [ CoCl4]2– Spectroscopic ground state term symbols of cobalt ions in [Co(H2O)6]2+ and [ CoCl4]2–](https://www.gkseries.com/blog/wp-content/uploads/2023/09/Spectroscopic-ground-state-term-symbols-of-cobalt-ions-in-CoH2O62-and-CoCl42%E2%80%93.jpg)
![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2 Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure-Parmagnetic-Diamagnetic-Examples-dsp2](http://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-3.png)
![Odia] Write the IUPAC name of the followings. K2 [ CoCl4] Odia] Write the IUPAC name of the followings. K2 [ CoCl4]](https://static.doubtnut.com/ss/web/10807229.webp)